In chemistry, a lactam is a cyclic amide. The name is derived from two chemical terms, lactone, referring to a cyclic ketone, and amide, a compound containing a nitrogen atom next to a carbonyl group. Lactams are named according to the size of the cyclic ring in the lactam:  -lactams,
-lactams,  -lactams,
-lactams,  -lactams and
-lactams and  -lactams contain rings made of three, four, five or six atoms, respectively.
-lactams contain rings made of three, four, five or six atoms, respectively.  -lactams are also called aziridinones. Many widely used antibiotic drugs, including the penicillins and cephalosporins, owe their activity to the presence of a
-lactams are also called aziridinones. Many widely used antibiotic drugs, including the penicillins and cephalosporins, owe their activity to the presence of a  -lactam structure. The lactams may have substitutions added to the nitrogen atom or any of the non-carbonyl carbon atoms in the base structure.
-lactam structure. The lactams may have substitutions added to the nitrogen atom or any of the non-carbonyl carbon atoms in the base structure.
Synthesis
General synthetic methods exist for the organic synthesis of lactams.
- Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
- Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction.
- Lactams form from cyclisation of amino acids.
- Lactams form from intramolecular attack of linear acyl derivatives from the nucleophilic abstraction reaction.
- In iodolactamization an iminium ion reacts with an halonium ion formed in situ by reaction of an alkene with iodine.
- L actams form by copper catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
- Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both β- as well as γ-lactam. At lower temp (−78 °C) β-lactam is the preferred product. At optimum temperatures, a highly useful γ-lactam known as Vince Lactam is obtained
Beckmann Rearrangement
An acid-induced rearrangement of oximes to give amides.
This reaction is related to the Hofmann and Schmidt Reactions and the Curtius Rearrangement, in that an electropositive nitrogen is formed that initiates an alkyl migration.
Mechanism of the Beckmann Rearrangement
Oximes generally have a high barrier to inversion, and accordingly this reaction is envisioned to proceed by protonation of the oxime hydroxyl, followed by migration of the alkyl substituent "trans" to nitrogen. The N-O bond is simultaneously cleaved with the expulsion of water, so that formation of a free nitrene is avoided.
Schmidt Reaction
Mechanism of the Schmidt Reaction
Reaction of carboxylic acids gives acyl azides, which rearrange to isocyanates, and these may be hydrolyzed to carbamic acid or solvolysed to carbamates. Decarboxylation leads to amines.
The reaction with a ketone gives an azidohydrin intermediate, which rearranges to form an amide:
Alkenes are able to undergo addition of HN3 as with any HX reagent, and the resulting alkyl azide can rearrange to form an imine:
Tertiary alcohols give substitution by azide via a carbenium ion, and the resulting alkyl azide can rearrange to form an imine.
Kinugasa reaction
General structure of a nitrone
A nitrone is the N-oxide of an imine and a functional group in organic chemistry. The general structure is R1R2C=NR3+O- where R3 is different from H.
A nitrone is 1,3-dipole in 1,3-dipolar cycloadditions. It reacts with alkenes to form an isoxazolidine:
One example of this reaction type is the reaction of various Baylis-Hillman adducts with C-Phenyl-N-methylnitrone forming an isoxazolidine in which R1 is phenyl, R2 is hydrogen and R3 is a methyl group .
Nitrones react with terminal alkynes and a copper salt to beta-lactam. This reaction is also called The Kinugasa reaction for example in this reaction:
The first step in this reaction is a dipolar cycloaddition of the nitrone with the in situ generated copper(I) acetylide to a 5-membered ring structure which rearranges in the second step.
My Problem
Why is the β-lactams are more reactive to hydrolysis conditions than are linear amides or larger lactams?
This strain is further increased by fusion to a second ring, as found in most β-lactam antibiotics. This trend is due to the amide character of the β-lactam being reduced by the aplanarity of the system. The nitrogen atom of an ideal amide is sp2-hybridized due to resonance, and sp2-hybridized atoms have trigonal planar bond geometry. As a pyramidal bond geometry is forced upon the nitrogen atom by the ring strain, the resonance of the amid bond is reduced, and the carbonyl becomes more ketone-like. Nobel laureate Woodward described a parameter h as a measure of the height of the trigonal pyramid defined by the nitrogen (as the apex) and its three adjacent atoms. h corresponds to the strength of the β-lactam bond with lower numbers (more planar; more like ideal amides) being stronger and less reactive. Monobactams have h values between 0.05 and 0.10 angstroms (Å). Cephems have h values in of 0.20–0.25 Å. Penams have values in the range 0.40–0.50 Å, while carbapenems and clavams have values of 0.50–0.60 Å, being the most reactive of the β-lactams toward hydrolysis.

















7 komentar:
10 Juni 2012 pukul 05.41
great explanation, vebria. but i wanna ask something that i didn't understand.
how the mechanism of amydes to be cyclic, and then form the lactams?
please answer it to my blog. thanks cantik!;)
10 Juni 2012 pukul 07.06
Debi: Thanks so much :D semoga bermanfaat
10 Juni 2012 pukul 07.20
I think , it’s because ring structure from beta lactam not bond to second ring in the molecule. So that it isn’t form crosswise bond and peptidoglican form not perfect so that weaker and easy degradation.
If beta lactam more reactive in hydrolisis condition,so how is with lactam itself ? What is lactam more reactive,too??
10 Juni 2012 pukul 08.05
Rara Cantik: according the refferences that I read,I will try to answer that "An amide may be produced by combining a carboxylic acid with an amino group. In addition to amide formation, a molecule of water is related.
Lactams - Cyclic Amides
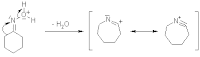
With some modification, a reaction may be carried out that generates a ring structure containing a similar linkage. Consider the result if two functional groups are a distance apart on the same molecule and react together. Consider, for instance, 4-aminobutyric acid, H2N-CH2CH2CH2-COOH. If reacted utilizing the proper conditions,2
H2N-CH2CH2CH2-COOH → 5-member ring + HOH (see associated image). The amino group at one end reacts with the carboxylic acid group at the other, closing the molecule to form the ring. The ring structure is called a lactam"
10 Juni 2012 pukul 08.36
The rate of anionic lactam polymerization is greatly affected by variation of the permittivity of lactams with ring size and substitution, as well as by changes of permittivity during polymerization. Therefore, an estimation of reactivities under comparable conditions is possible in solution only. The lack of any solvent permitting anionic lactam polymerization at low temperatures was circumvented by using living polymers soluble in aprotic solvents as carriers. Such polymers are able to remain in solution even after the addition of a few monomer units of a lactam, the polymer of which is insoluble. In this way, relative reactivities of a series of four-membered lactams, as well as that of the five-membered one were established.
12 Juni 2012 pukul 09.13
You've posted good topic but why don't you post about application of lactam? as i know lactam has much application in our life especially in medicine
12 Juni 2012 pukul 10.39
Thanks your sugestion Yani, actually there are much application of lactam. One of it is β-Lactam antibiotics (beta-lactam antibiotics) are a broad class of antibiotics, consisting of all antibiotic agents that contains a β-lactam nucleus in their molecular structures. This includes penicillin derivatives (penams), cephalosporins (cephems), monobactams, and carbapenems.[1] Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics.
I think it is too long if i explain here. I'll give some link about it http://amrls.cvm.msu.edu/pharmacology/antimicrobials/antibiotics-of-veterinary-importance/beta-lactam-antibiotics
http://en.wikipedia.org/wiki/Penicillin
http://en.wikipedia.org/wiki/Beta-lactam_antibiotic
Posting Komentar